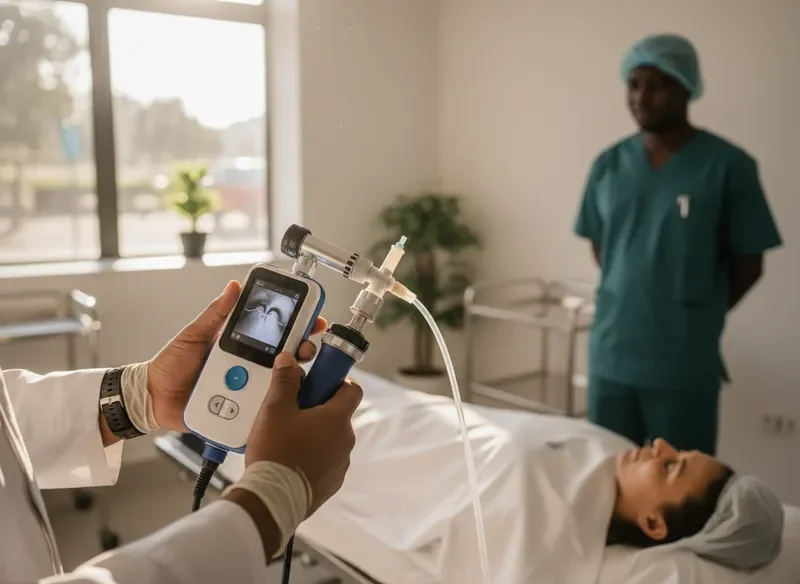
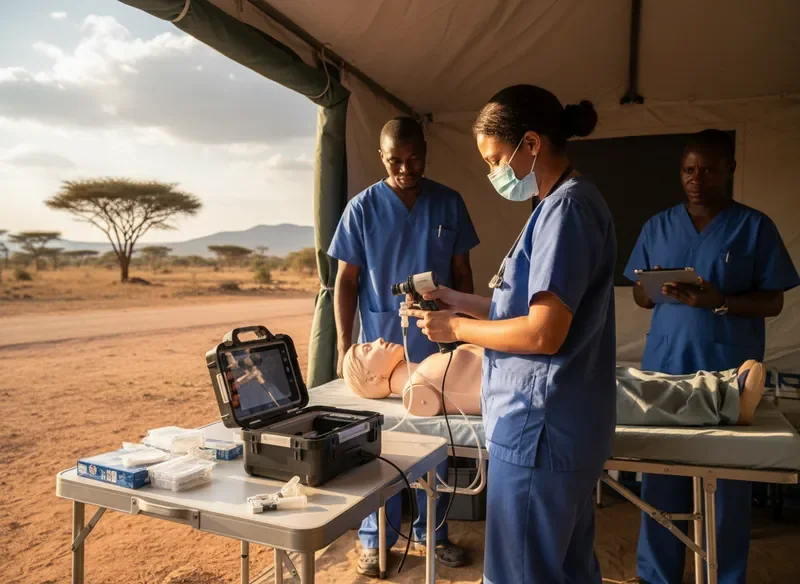
Emergency Department Integration – Nairobi Teaching Hospital
A 400-bed tertiary facility introduced our laryngoscopy system into their emergency airway protocol. The transition from legacy equipment required minimal retraining, and staff reported increased confidence during critical intubations within the first month.
Observed workflow improvement:Average intubation preparation time reduced by 40%, allowing faster response in trauma cases where every second counts.
User feedback:Senior anesthesiologists noted the ergonomic handle design reduced hand fatigue during prolonged procedures, particularly in overnight shifts.
Training note:Junior residents achieved proficiency with the device in half the expected time compared to previous equipment, attributed to the intuitive blade design and clear visualization.